
Because each carbon in acetylene has two electron groups, VSEPR predicts a linear geometry and and H-C-C bond angle of 180 o. Draw a second structure with bonds drawn. Using Lewis Structures and the VSEPR model, predict the molecular geometries of CO2, CH4, C2H2,C2H4, and C2H6 and then use the.
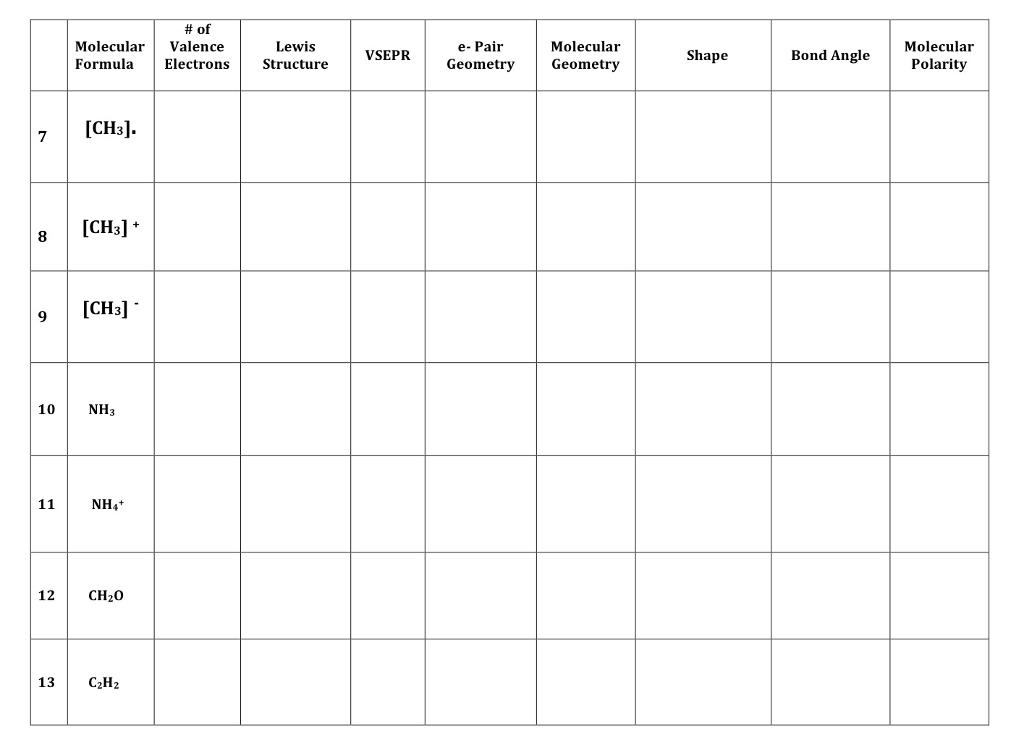
State if the molecule is polar or not polar. Using Lewis Structures and the VSEPR model, predict the molecular geometries of CO2, CH4, C2H2,C2H4, and C2H6 and then use the geometries to describe the hybridization (sp, sp2, or sp3) expected for each carbon atom. Identify the VSEPR notation, shape, and hybridization of the central atom. The carbon-carbon triple bond in acetylene is the shortest (120 pm) and the strongest (965 kJ/mol) of the carbon-carbon bond types. Include major resonance forms with formal charges.
C2H2 VSEPR PLUS
These two perpendicular pairs of p orbitals form two pi bonds between the carbons, resulting in a triple bond overall (one sigma bond plus two pi bonds).Īcetylene is said to have three sigma bonds and two pi bonds. The molecule or ions structure is determined by the VSEPR theory (Valence Shell Electron Pair Repulsion Theory), based on the molecular geometry by knowing. Each carbon atom still has two half-filled 2 p y and 2 p z orbitals, which are perpendicular both to each other and to the line formed by the sigma bonds. The C-C sigma bond is formed by the overlap of one sp orbital from each of the carbons, while the two C-H sigma bonds are formed by the overlap of the second sp orbital on each carbon with a 1 s orbital on a hydrogen. The 2 p y and 2 p z orbitals remain non-hybridized, and are oriented perpendicularly along the y and z axes, respectively. In an sp-hybridized carbon, the 2 s orbital combines with the 2 p x orbital to form two sp hybrid orbitals that are oriented at an angle of 180°with respect to each other (eg. In the hybrid orbital picture of acetylene, both carbons are sp-hybridized.

The carbon-carbon triple bond is only 1.20Å long. In the C 2 H 2 Cl 2 lewis structure, there are 4 single bonds, one double bond, and a total of 6 lone pairs are present. The molecular geometry of C 2 H 2 Cl 2 for both carbon central atoms is Trigonal planar. We must first draw the Lewis structure for CHO. The total valence electron is available for drawing the C2H2Cl2 lewis structure is 24. This molecule is linear: all four atoms lie in a straight line. The VSEPR model predicts that CHO is trigonal planar with bond angles of about 120 °. Consider, for example, the structure of ethyne (another common name is acetylene), the simplest alkyne. \)įinally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles. For the hydronium cation (HO):a) Draw the Lewis structure from its elements.b) Predict the shape of the ion using VSEPR theory.c) Draw the ion in perspecti.


 0 kommentar(er)
0 kommentar(er)
